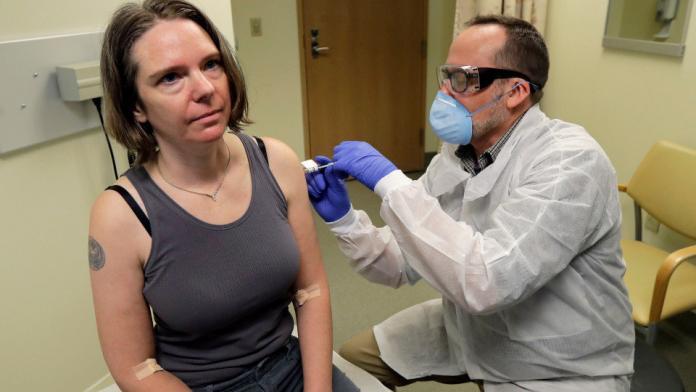
The first human trial has commenced today to create a vaccine to protect against the coronavirus. A group of 45 healthy volunteers received the trial vaccine at a Kaiser Permanente research facility in Seattle, WA. Injections will be given to each volunteer over a six-week period and will take months to determine if it will be effective in combating the virus.
“The first participant received the investigational vaccine today,” the US National Institutes of Health (NIH) said in a statement on Monday.
Participants will receive different doses of the experimental vaccine and receive two separate doses 28 days apart. It’s important to understand that even if these first trials are a success the general public may still have to wait upwards of 18 months for a legitimate vaccine to be made available.
These human trials were fast-tracked due to the National Institutes of Health funding the trial, which helped sidestep a variety of existing laws a red tape that would normally have been conducted.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said the trial vaccine contains lab-produced genetic material that contains a harmless genetic code copied from the COVID-19 virus that actually causes the disease. Much like any vaccine, the flu vaccine being a great example, the theory behind these trials is to introduce a part of the genetic code of the disease to help people’s bodies figure out ways to fight it and create immunities to it.




